November 26, 2024 by Chris Cesare, Joint Quantum Institute
Collected at: https://phys.org/news/2024-11-electrons-quantum-interactions.html
It’s hard to tell when you’re catching some rays at the beach, but light packs a punch. Not only does a beam of light carry energy, it can also carry momentum. This includes linear momentum, which is what makes a speeding train hard to stop, and orbital angular momentum, which is what the Earth carries as it revolves around the sun.
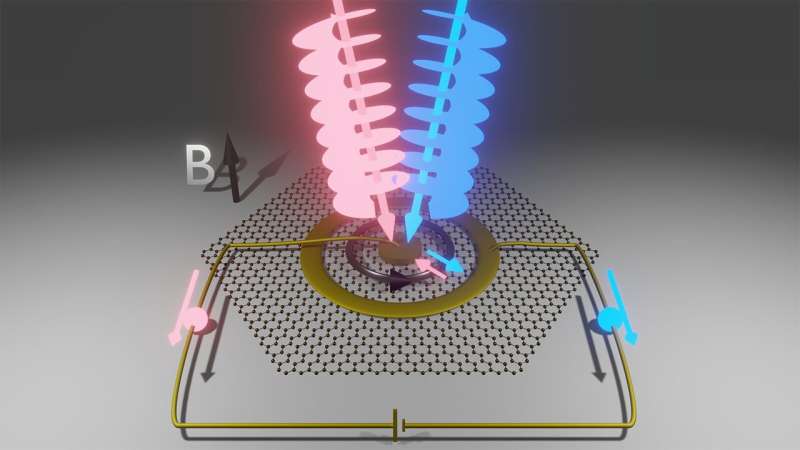
In a new paper, scientists seeking better methods for controlling the quantum interactions between light and matter have demonstrated a novel way to use light to give electrons a spinning kick. They reported the results of their experiment, which shows that a light beam can reliably transfer orbital angular momentum to itinerant electrons in graphene, on Nov. 26, 2024, in the journal Nature Photonics.
Having tight control over the way that light and matter interact is an essential requirement for applications like quantum computing or quantum sensing. In particular, scientists have been interested in coaxing electrons to respond to some of the more exotic shapes that light beams can assume.
For example, light carrying orbital angular momentum swirls around its axis as it travels. When viewed head-on, a light beam with orbital angular momentum contains a dark spot in the middle, a vortex opened up by the beam’s corkscrew character.
“The interaction of light that has orbital angular momentum with matter has been thought about since the 90s or so,” says Deric Session, a postdoctoral researcher at JQI and the University of Maryland (UMD) who is the lead author of the new paper. “But there have been very few experiments actually demonstrating the transfer.”
Part of the challenge has been a size mismatch. In order for electrons to feel a tug from a light beam carrying momentum, they have to experience the way that the beam changes as it passes by. In many cases, the length over which a light beam changes dwarfs the size of the matter that researchers are interested in manipulating, making it especially challenging to pick out electrons as targets.
For instance, atoms and their orbiting electrons—mainstays of quantum physics experiments and favorite targets for precise manipulation—are roughly 1,000 times smaller than the light beams that researchers use to interact with them. Light travels as repeating waves of electric and magnetic fields, and the length that a light beam travels before it repeats is called the wavelength.
In addition to being an important characteristic size, the wavelength of light also determines the amount of energy carried by individual particles of light called photons. Only photons that carry particular amounts of energy can interact with atoms, and those photons tend to have wavelengths much bigger than the atoms themselves.
So, while atoms as a whole will happily absorb energy and momentum from these photons, the wavelength is too big for the internal pieces of the atom—the nucleus and the electrons—to notice any relative difference. This makes it very difficult to transfer orbital angular momentum solely to an atom’s electrons.
One way to overcome this difficulty is to shrink the wavelength of light. But that increases the energy carried by each photon, ruling out atoms as reliable targets. In the new experiment, the researchers, who included JQI Fellows Mohammad Hafezi and Nathan Schine, JQI Co-Director Jay Sau and JQI Adjunct Fellow Glenn Solomon, pursued an alternate approach: Instead of shrinking the wavelength of the light, they puffed up electrons to make them occupy more space.
Electrons bound to the nucleus of an atom can only roam so far before they are liberated from the atom and useless for experiments. But in conductive materials, electrons have more latitude to travel far and wide while remaining under control. The researchers turned to graphene, a flat material that is one of the best known electrical conductors, in search of a way to make electrons take up more space.
By cooling a sample of graphene down to just 4 degrees above absolute zero and subjecting it to a strong magnetic field, electrons that are ordinarily free to move around become trapped in loops called cyclotron orbits. As the field gets stronger, the orbits become tighter and tighter until many circulating electrons are packed in so tightly that no more can fit. Although the orbits are tight, they are still much larger than the electron orbitals in atoms—the perfect recipe for getting them to notice light carrying orbital angular momentum.
The researchers used a sample of graphene wired up with electrodes for their experiments. One electrode was in the middle of the sample, and another made a ring around the outer edges.
Earlier theoretical work, developed in 2021 by former JQI and UMD graduate student Bin Cao and three other authors of the new paper, suggested that electrons circulating in such a sample could gain angular momentum in chunks from incoming light, increase the size of their orbits and eventually get absorbed by the electrodes.
“The idea is that you can change the size of the cyclotron orbits by adding or subtracting orbital angular momentum from the electrons, thus effectively moving them across the sample and creating a current,” Session says.
In the new paper, the research team reported observing a robust current that survived under a wide range of experimental conditions. They hit their graphene sample with light carrying orbital angular momentum that circulated clockwise and observed the current flowing in one direction.
Then they hit it with light carrying counterclockwise orbital angular momentum and found that the direction of the current flipped. They flipped the direction of the applied magnetic field and observed the current flip directions, too—an expected finding since changing the magnetic field direction also swaps the direction electrons flow in their cyclotron orbits. They changed the voltage across the inner and outer electrodes and continued to see the same difference between currents generated by clockwise and counterclockwise vortex light.
They also tested sending circularly polarized light, which carries an intrinsic angular momentum, at the sample, and they found that it barely generated any current. In all cases, the signal was clear: The current only appeared in the presence of light carrying orbital angular momentum, and the direction of the current was correlated with whether the light carried momentum that spun clockwise or counterclockwise.
The result was the culmination of several years of work, which included some false starts with sample fabrication and difficulties collecting enough good data from the experiment.
“I spent over a year just trying to make graphene samples with this kind of geometry,” Session says. Ultimately, Session and the team reached out to a group they had worked with before, led by Roman Sordan, a physicist at the Polytechnic University of Milan in Italy and an expert at preparing graphene samples.
“They were able to come through and make the samples that we used,” says Session.
Once they had samples that worked well, they still had trouble aligning their twisted light with the sample to observe the current.
“The signal we were looking at was not quite consistent,” says Mahmoud Jalali Mehrabad, a postdoctoral researcher at JQI and UMD and a co-author of the paper. “Then one day, with Deric, we started to do this spatial sweep. And we kind of mapped the sample with really high accuracy. Once we did that—once we nailed down the very peak, optimized position for the beam—everything started to make sense.”
Within a week or so, they had collected all the data they needed and could pick out all the signals of the current’s dependence on the orbital angular momentum of the beam.
Mehrabad says that, in addition to demonstrating a new method for controlling matter with light, the technique might also enable fundamentally new measurements of electrons in quantum materials. Specially prepared light beams, combined with interference measurements, could be used as a microscope that can image the spatial extent of electrons—a direct measurement of the quantum nature of electrons in a material.
“Being able to measure these spatial degrees of freedom of free electrons is an important part of measuring the coherence properties of electrons in a controllable manner—and manipulating them,” Mehrabad says. “Not only do you detect, but you also control. That’s like the holy grail of all this.”
More information: Deric Session et al, Optical pumping of electronic quantum Hall states with vortex light, Nature Photonics (2024). DOI: 10.1038/s41566-024-01565-1
Journal information: Nature Photonics

Leave a Reply