September 3, 2024 by Ingrid Fadelli , Tech Xplore
Collected at: https://techxplore.com/news/2024-09-catalyst-boosts-anion-exchange-membrane.html
Fuel cells are energy-conversion solutions that generate electricity via electrochemical reactions without combustion, thus not contributing to the pollution of air on Earth. These cells could power various technologies, ranging from electric vehicles to portable chargers and industrial machines.
Despite their advantages, many fuel cell designs introduced to date rely on expensive materials and precious metal catalysts, which limits their widespread adoption. Anion-exchange-membrane fuel cells (AEMFCs) could help to tackle these challenges, as they are based on Earth-abundant, low-cost catalysts and could thus be more affordable.
In recent years, many research groups worldwide have been designing and testing new AEMFCs. While some existing devices achieved promising results, most of the non-precious metals serving as catalysts were found to be prone to self-oxidation, which causes the irreversible failure of the cells.
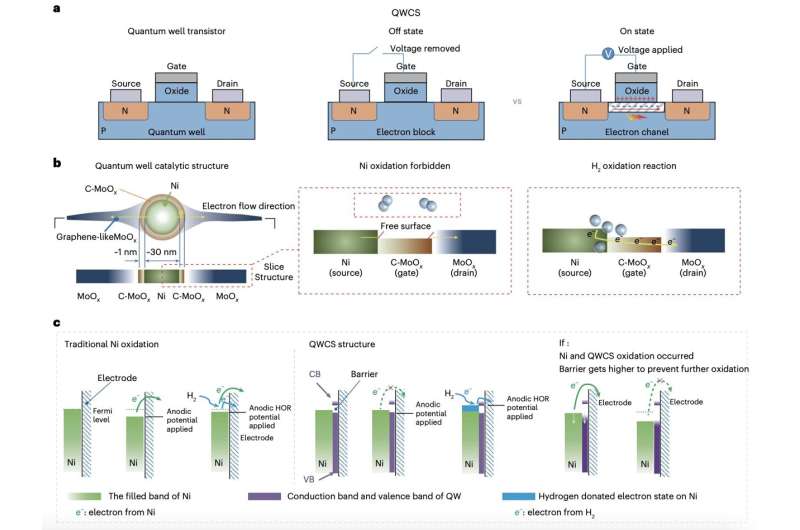
Researchers at Chongqing University and Loughborough University have recently devised a strategy that could prevent the oxidation of metallic nickel electrocatalysts for AEMFCs. This strategy, introduced in a paper in Nature Energy, entails the use of a newly designed quantum well-like catalytic structure (QWCS) consisting of quantum-confined metallic nickel nanoparticles.
“Non-precious metals used in AEMFCs to catalyze the hydrogen oxidation reaction are prone to self-oxidation, resulting in irreversible failure,” Yuanyuan Zhou, Wei Yuan and their colleagues wrote in their paper. “We show a QWCS, constructed by atomically confining Ni nanoparticles within a carbon-doped-MoOx/MoOx heterojunction (C-MoOx/MoOx) that can selectively transfer external electrons from the hydrogen oxidation reaction while remaining itself metallic.”
QWCSs are nanostructures displaying quantum well properties that can enhance catalytic activity. The new QWCS constructed by the researchers is comprised of Ni nanoparticles atomically confined into a heterojunction consisting of crystallized carbon-doped MoOx (C-MoOx) as the low energy valley and amorphous MoOx as the high energy barrier.
The catalyst they designed, called Ni@C-MoOx, can selectively transfer external electrons produced via the catalysis of the hydrogen oxidation reaction without transferring electrons from the Ni catalyst into the QWCS’ valley. This selective transfer of electrons makes the catalyst robust against electro-oxidation, protecting fuel cells from degradation and failure.
The Ni@C-MoOx catalyst, which was found to sustain excellent HOR catalytic stability after 100 hours of continuous operation under harsh conditions, was used to create an anode-catalyzed alkaline fuel cell. This fuel cell attained remarkable results, exhibiting a high specific power density of 486 mW mgNI-1, with no decline in performance following repeated shutdown-start cycles.
“Electrons of Ni nanoparticles gain a barrier of 1.11 eV provided by the QWCS leading to Ni stability up to 1.2 V versus the reversible hydrogen electrode (VRHE) whereas electrons released from the hydrogen oxidation reaction easily cross the barrier by a gating operation of QWCS upon hydrogen adsorption,” wrote Zhou, Yuan and their colleagues. “The QWCS-catalyzed AEMFC achieved a high-power density of 486 mW mgNi−1 and withstood hydrogen starvation operations during shutdown–start cycles, whereas a counterpart AEMFC without QWCS failed in a single cycle.”
The new catalytic structure designed and constructed by this team of researchers could soon contribute to the development of cost-effective AEMFCs that are more reliable and do not degrade rapidly over time. Its underlying design strategy could also be used to create other promising catalysts that leverage quantum confinement to prevent the electro-oxidation of non-precious metals.
More information: Yuanyuan Zhou et al, Quantum confinement-induced anti-electrooxidation of metallic nickel electrocatalysts for hydrogen oxidation, Nature Energy (2024). DOI: 10.1038/s41560-024-01604-9
Journal information: Nature Energy

Leave a Reply