By Ashwini Sakharkar 12 Dec, 2024
Collected at: https://www.techexplorist.com/new-technique-enables-laser-light-penetrate-deeper-living-tissue/94505/
Metabolic imaging offers a noninvasive approach for clinicians and scientists to investigate living cells using laser light, significantly enhancing the assessment of disease progression and treatment efficacy. However, the effectiveness of this method faces challenges due to light scattering in biological tissues, which restricts penetration depth and reduces image resolution.
Recently, researchers from MIT have unveiled an innovative technique that dramatically expands the depth limit of metabolic imaging, more than doubling its reach while also increasing the speed of imaging. This cutting-edge method eliminates the need to alter the tissue, providing a more natural and accurate representation of its structure and function.
By utilizing a specialized laser that penetrates deep into the tissue, specific intrinsic molecules emit light without altering the tissue. Researchers achieed this by adaptive customization of the laser light for deep tissue imaging.
Through the use of a novel fiber shaper—a device they control by bending it—the researchers can finely tune the laser’s color and pulse duration, effectively reducing scattering and maximizing the signal’s strength as it penetrates deeper into the tissue. These improvements in penetration depth, speed, and resolution position the technique as particularly advantageous for sophisticated imaging tasks, including cancer research, tissue engineering, drug discovery, and the investigation of immune responses.
“This work shows a significant improvement in terms of depth penetration for label-free metabolic imaging. It opens new avenues for studying and exploring metabolic dynamics deep in living biosystems,” says Sixian You, assistant professor in the Department of Electrical Engineering and Computer Science (EECS), a member of the Research Laboratory for Electronics, and senior author of a paper on this imaging technique.
This new method falls in the category of label-free imaging, which means tissue is not stained beforehand. Traditional staining enhances contrast that helps a clinical biologist see cell nuclei and proteins better. However, this process usually involves cutting and slicing the sample, which often destroys the tissue and restricts the ability to observe dynamic processes in living cells.
On the other hand, in label-free imaging techniques, researchers use lasers that illuminate specific molecules within cells, prompting them to emit light in various colors that disclose different molecular components and cellular structures.
Despite this potential, generating the ideal laser light with precise wavelengths and high-quality pulses for deep-tissue imaging has posed significant challenges. To address this limitation, the researchers have introduced an innovative approach. They employ a multimode fiber, a powerful type of optical fiber, in conjunction with a compact device known as a “fiber shaper.” This shaper enables them to precisely control light propagation by adaptively altering the shape of the fiber.
By bending the fiber, they can modify the color and intensity of the laser light. Building upon earlier advancements, the researchers have refined the first iteration of the fiber shaper to facilitate deeper multimodal metabolic imaging.
“We want to channel all this energy into the colors we need with the pulse properties we require. This gives us higher generation efficiency and a clearer image, even deep within tissues,” says Cao.
After successfully engineering the controllable mechanism, they created an imaging platform that leverage the powerful laser source to generate longer wavelengths of light, essential for penetrating biological tissues more effectively.
“We believe this technology has the potential to significantly advance biological Research. By making it affordable and accessible to biology labs, we hope to empower scientists with a powerful tool for discovery,” Liu says.
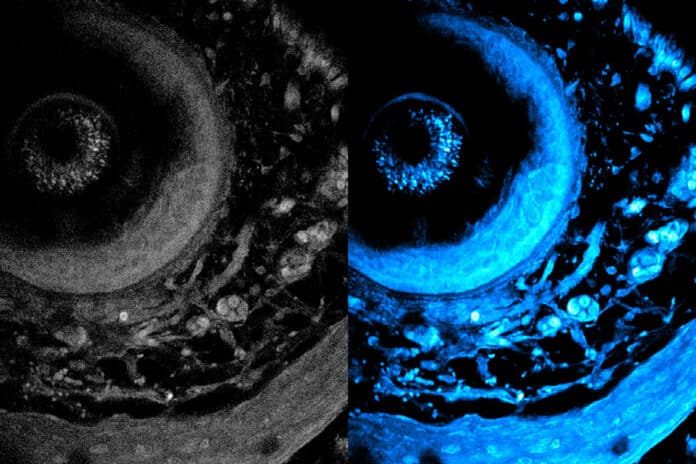
When the researchers tested their imaging device, the light was able to penetrate more than 700 micrometers into a biological sample, whereas the best prior techniques could only reach about 200 micrometers. The deep imaging approach allowed them to see cells at multiple levels within a living system, which could help researchers study metabolic changes that happen at different depths.
Moreover, the increased imaging speed allows for more comprehensive data on how a cell’s metabolism influences the velocity and direction of its movements. This novel imaging technique could significantly enhance the Research on organoids, which are engineered cells designed to replicate the structure and function of organs.
Researchers in the Kamm and Griffith labs pioneered the development of brain and endometrial organoids that can grow like organs to assess disease and treatment.
Despite the difficulty of accurately observing internal processes without damaging or staining the tissue, which can compromise the sample, this novel imaging technique enables scientists to observe noninvasively the metabolic conditions within a living organoid as it grows.
With these and additional biomedical uses in mind, the researchers aspire to achieve even sharper images. Simultaneously, they are focused on developing low-noise laser sources, which could facilitate deeper imaging with reduced light exposure.
Additionally, they are working to create low-noise laser sources, which could enable deeper imaging with less light dosage. They are also developing algorithms that react to the images to reconstruct the full 3D structures of biological samples in high resolution. In the long run, they hope to apply this technique in the real world to help biologists monitor drug response in real-time and aid in the development of new medicines.
“By enabling multimodal metabolic imaging that reaches deeper into tissues, we’re providing scientists with an unprecedented ability to observe nontransparent biological systems in their natural state. We’re excited to collaborate with clinicians, biologists, and bioengineers to push the boundaries of this technology and turn these insights into real-world medical breakthroughs,” You say.
“This work is exciting because it uses innovative feedback methods to image cell metabolism deeper in tissues compared to current techniques. These technologies also provide fast imaging speeds, which were used to uncover unique metabolic dynamics of immune cell motility within blood vessels. I expect that these imaging tools will be instrumental for discovering links between cell function and metabolism within dynamic living systems,” says Melissa Skala, an investigator at the Morgridge Institute for Research who was not involved with this work.
Journal reference:
- Kunzan Liu, Honghao Cao, Kasey Shashaty, Li-Yu Yu, Sarah Spitz, Francesca Michela Pramotton, Zhengpeng Wan, Ellen L. Kan, Erin N. Tevonian, Manuel Levy, Eva Lendaro, Roger D. Kamm, Linda G. Griffith, Fan Wang, Tong Qiu, Sixian You. Deep and dynamic metabolic and structural imaging in living tissues. Science Advances, 2024; DOI: 10.1126/sciadv.adp2438

Leave a Reply