December 2, 2024 by Kimm Fesenmaier, California Institute of Technology
Collected at: https://phys.org/news/2024-12-quantum-ultrafast-laser-pulses-class.html
In the effort to develop new quantum technologies of the future, scientists are pursuing several different approaches. One avenue seeks to use molecules as the fundamental building blocks of quantum technologies.
Now scientists at Caltech have figured out a new way to use ultrafast laser pulses to realize an important quantum mechanical property known as superposition, turning a relatively simple molecule into a quantum sensor—a tool that can measure chemical phenomena in its surroundings through inherently quantum means.
Superposition is commonly understood using what is known as the Schrödinger’s cat thought experiment, which posits that a cat in a box could exist as simultaneously dead and alive until an observation or measurement of the system is made. Similarly, an electron in a superposition exists in multiple possible states, each representing a different outcome with a different probability of being observed once a measurement is taken.
At the heart of many quantum technologies are what are known as quantum bits, or qubits, the quantum analogs of the bits in classical computers (the ones we all use today). Unlike classical bits, however, qubits (which might be molecules, atoms, photons, or electrons, for example, depending on the quantum computing/sensing system) can exhibit this bizarre phenomenon of superposition.
Because qubits can simultaneously exist in multiple states, they can have exponentially more computing power than classical bits. However, a superposition will rapidly collapse into one of its constituent states through interaction with its surroundings, creating a technological challenge that must be overcome before devices such as quantum computers can be fully realized.
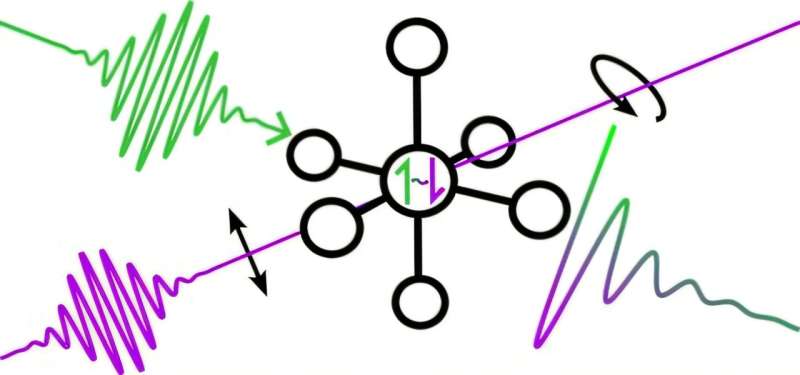
In a paper recently published in the journal Science, researchers in the lab of Ryan G. Hadt, assistant professor of chemistry at Caltech, describe how a class of molecules can be used in combination with femtosecond pulses of light (lasting just a few millionths of a billionth of a second) to measure instances of superposition at room temperature.
Specifically, they show how to measure electron spin superpositions, a quantum mechanical property that determines the direction of a magnetic field produced by an electron.
“This technique could allow for the study of biological systems or materials or other types of chemical processes in ways that you could not have done before,” says Hadt.
The work, led by Erica Sutcliffe, a postdoctoral scholar in chemistry, takes advantage of the electronic structure of a relatively simple molecule: potassium hexachloroiridate (IV), K2IrCl6.
K2IrCl6 is basically an ion of iridium surrounded by six symmetrically distributed chloride ions, but it is also an example of what is called a paramagnetic molecule, or one that has unpaired electrons.
“In all molecules, electrons can only sit in very specific states, but, in highly-symmetric paramagnetic molecules like this, these states are arranged such that we can efficiently manipulate the electron spin with light,” explains Sutcliffe.
The researchers use a technique called pump-probe polarization spectroscopy to create and then track superpositions of electron spins. They irradiate a sample of K2IrCl6 in water with a femtosecond laser pulse. This laser pulse is carefully chosen to have a particular polarization (the polarization of light represents the orientation of propagating light waves as they oscillate, with respect to their direction of travel).
“If we choose the correct polarization of the light, our regular molecules that are in equilibrium are put into a spin superposition,” Sutcliffe says. The laser pulse moves an electron from one state to a higher, or excited, one, and that generates the superposition.
After a few tiny fractions of a second, the researchers pass another weaker laser pulse through the sample and measure how the polarization of the light has changed. By continuing to take measurements in this way, they can determine how long the electrons remain in a superposition before they relax back to their starting state.
“Not just any molecule can be measured in this way,” says Nathanael P. Kazmierczak, a graduate student in Hadt’s lab and a co-author of the paper. “So there were two key insights here: One was developing the instrumental technique and one was finding the design principle for the molecules that would work with this type of instrument.”
While the Caltech team is the first to show that a molecule with paramagnetic properties can be used to initiate and measure electron spin superposition in this manner, Sutcliffe says the technique is not specific to K2IrCl6. “We don’t anticipate that the molecule we found is the best,” she says. “Rather, it is an example of a whole new class of molecular probes for quantum properties in these systems.”
In addition to being useful for studying superposition and how long it can be sustained, the molecules can also be used as quantum sensors. For example, the electron superposition is sensitive to various chemical properties such as the viscosity of the molecule’s immediate surroundings or the presence of common nuclei that generate their own magnetic field.
The simplicity of the approach also enables it to be broadly useful, Sutcliffe says. “Because we are only using light in this technique, and we don’t have to use a really big magnet or microwaves as with other methods, we can measure properties on really, really fast timescales and also at small size scales.
“That means we could potentially do microscopy with this technique, which hasn’t previously been accessible, opening up studies of previously underexplored properties in biological systems.”
Furthermore, the team says it might be possible to use electron superpositions to identify individual mutations in proteins. “Given the sensitivity of the superposition to the spatial distribution of other nuclei, it’s reasonable to ask how does protein structure and amino acid composition affect a spin superposition?” says Hadt.
“If we can learn something about that, we might be able to provide information as to whether or not certain cancerous point mutations exist in a protein.”
More information: Erica Sutcliffe et al, Ultrafast all-optical coherence of molecular electron spins in room-temperature water solution, Science (2024). DOI: 10.1126/science.ads0512
Journal information: Science

Leave a Reply