October 23, 2024 by University of California – Berkeley
Collected at: https://techxplore.com/news/2024-10-covalent-framework-quickly-captures-ambient.html
Capturing and storing the carbon dioxide humans produce is key to lowering atmospheric greenhouse gases and slowing global warming, but today’s carbon capture technologies work well only for concentrated sources of carbon, such as power plant exhaust. The same methods cannot efficiently capture carbon dioxide from ambient air, where concentrations are hundreds of times lower than in flue gases.
Yet direct air capture, or DAC, is being counted on to reverse the rise of CO2 levels, which have reached 426 parts per million (ppm), 50% higher than levels before the Industrial Revolution. Without it, according to the Intergovernmental Panel on Climate Change, we won’t reach humanity’s goal of limiting warming to 1.5 °C (2.7 °F) above preexisting global averages.
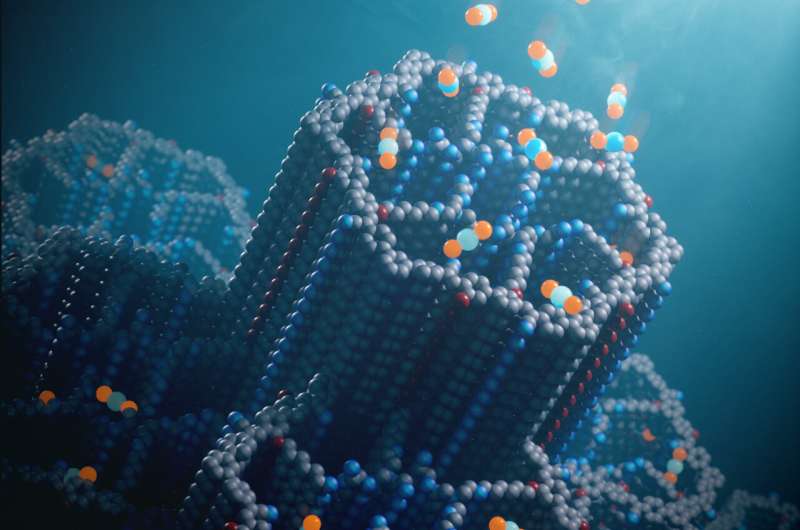
A new type of absorbing material developed by chemists at the University of California, Berkeley, could help get the world to negative emissions. The porous material—a covalent organic framework (COF)—captures CO2 from ambient air without degradation by water or other contaminants, one of the limitations of existing DAC technologies.
“We took a powder of this material, put it in a tube, and we passed Berkeley air—just outdoor air—into the material to see how it would perform, and it was beautiful. It cleaned the air entirely of CO2. Everything,” said Omar Yaghi, the James and Neeltje Tretter Professor of Chemistry at UC Berkeley and senior author of a paper that appeared online Oct. 23 in the journal Nature.
“I am excited about it because there’s nothing like it out there in terms of performance. It breaks new ground in our efforts to address the climate problem,” he added.
According to Yaghi, the new material could be substituted easily into carbon capture systems already deployed or being piloted to remove CO2 from refinery emissions and capture atmospheric CO2 for storage underground.
UC Berkeley graduate student Zihui Zhou, the paper’s first author, said that a mere 200 grams of the material, a bit less than half a pound, can take up as much CO2 in a year—20 kilograms (44 pounds)—as a tree.
“Flue gas capture is a way to slow down climate change because you are trying not to release CO2 to the air. Direct air capture is a method to take us back to like it was 100 or more years ago,” Zhou said.
“Currently, the CO2 concentration in the atmosphere is more than 420 ppm, but that will increase to maybe 500 or 550 before we fully develop and employ flue gas capture. So if we want to decrease the concentration and go back to maybe 400 or 300 ppm, we have to use direct air capture.”

COF vs. MOF
Yaghi is the inventor of COFs and MOFs (metal-organic frameworks), both of which are rigid crystalline structures with regularly spaced internal pores that provide a large surface area for gases to stick or adsorb.
Some MOFs that he and his lab have developed can adsorb water from the air, even in arid conditions, and when heated, release the water for drinking. He has been working on MOFs to capture carbon since the 1990s, long before DAC was on most people’s radar screens, he said.
Two years ago, his lab created a very promising material, MOF-808, that adsorbs CO2, but the researchers found that after hundreds of cycles of adsorption and desorption, the MOFs broke down. These MOFs were decorated inside with amines (NH2 groups), which efficiently bind CO2 and are a common component of carbon capture materials.
In fact, the dominant carbon capture method involves bubbling exhaust gases through liquid amines that capture the carbon dioxide. Yaghi noted, however, that the energy intensive regeneration and volatility of liquid amines hinders their further industrialization.
Working with colleagues, Yaghi discovered why some MOFs degrade for DAC applications—they are unstable under basic, as opposed to acidic, conditions, and amines are bases. He and Zhou worked with colleagues in Germany and Chicago to design a stronger material, which they call COF-999.
Whereas MOFs are held together by metal atoms, COFs are held together by covalent carbon-carbon and carbon-nitrogen double bonds, among the strongest chemical bonds in nature.
As with MOF-808, the pores of COF-999 are decorated inside with amines, allowing uptake of more CO2 molecules.
“Trapping CO2 from air is a very challenging problem,” Yaghi said.
“It’s energetically demanding, you need a material that has high carbon dioxide capacity, that’s highly selective, that’s water stable, oxidatively stable, recyclable. It needs to have a low regeneration temperature and needs to be scalable. It’s a tall order for a material. And in general, what has been deployed as of today are amine solutions, which are energy intensive because they’re based on having amines in water, and water requires a lot of energy to heat up, or solid materials that ultimately degrade with time.”
Yaghi and his team have spent the last 20 years developing COFs that have a strong enough backbone to withstand contaminants, ranging from acids and bases to water, sulfur and nitrogen, that degrade other porous solid materials.
The COF-999 is assembled from a backbone of olefin polymers with an amine group attached. Once the porous material has formed, it is flushed with more amines that attach to NH2 and form short amine polymers inside the pores. Each amine can capture about one CO2 molecule.

When 400 ppm CO2 air is pumped through the COF at room temperature (25 °C) and 50% humidity, it reaches half capacity in about 18 minutes and is filled in about two hours. However, this depends on the sample form and could be speeded up to a fraction a minute when optimized.
Heating to a relatively low temperature—60 °C, or 140 °F—releases the CO2, and the COF is ready to adsorb CO2 again. It can hold up to 2 millimoles of CO2 per gram, standing out from other solid sorbents.
Yaghi noted that not all the amines in the internal polyamine chains currently capture CO2, so it may be possible to enlarge the pores to bind more than twice as much.
“This COF has a strong chemically and thermally stable backbone, it requires less energy, and we have shown it can withstand 100 cycles with no loss of capacity. No other material has been shown to perform like that,” Yaghi said. “It’s basically the best material out there for direct air capture.”
Yaghi is optimistic that artificial intelligence can help speed up the design of even better COFs and MOFs for carbon capture or other purposes, specifically by identifying the chemical conditions required to synthesize their crystalline structures.
He is scientific director of a research center at UC Berkeley, the Bakar Institute of Digital Materials for the Planet (BIDMaP), which employs AI to develop cost-efficient, easily deployable versions of MOFs and COFs to help limit and address the impacts of climate change.
“We’re very, very excited about blending AI with the chemistry that we’ve been doing,” he said.
More information: Omar Yaghi, Carbon dioxide capture from open air using covalent organic frameworks, Nature (2024). DOI: 10.1038/s41586-024-08080-x. www.nature.com/articles/s41586-024-08080-x
Journal information: Nature

Leave a Reply