By Amit Malewar 11 Oct, 2024
Collected at: https://www.techexplorist.com/high-resolution-imaging-accessible/91080/
MIT researchers have created a method to expand tissue before imaging, allowing them to achieve nanoscale resolution with regular light microscopes instead of costly super-resolution microscopes.
In this latest version, they can expand tissue by 20 times in one step. This simple and affordable technique could make nanoscale imaging accessible to many biology labs.
Laura Kiessling, a professor at MIT, noted that this “democratizes imaging” by enabling high-resolution viewing without the need for expensive equipment. It allows scientists to see details that standard microscopes usually cannot capture, making imaging more cost-effective and widely available.
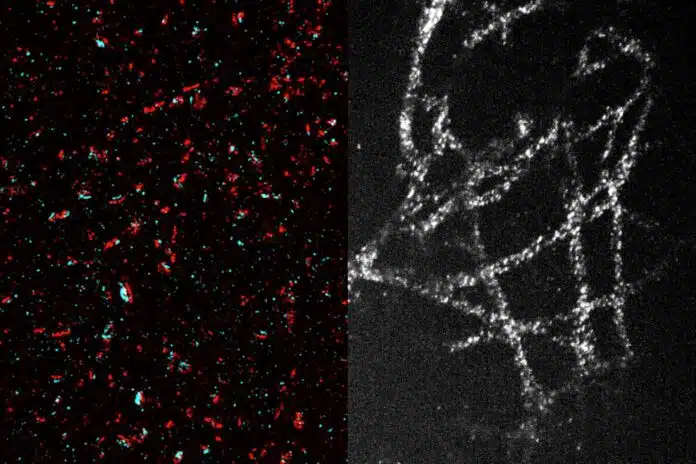
With this technique, scientists can achieve a resolution of about 20 nanometers, allowing them to see organelles inside cells and clusters of proteins. Twenty-fold expansion gets you into the realm that biological molecules operate in, making it possible to study structures at a scale relevant to biology.
The expansion microscopy was invented in 2015. The technique involves embedding tissue in an absorbent polymer and breaking down the proteins that hold the tissue together. When water is added, the gel expands, pulling biomolecules apart.
The original method allowed for about fourfold expansion, providing images with a resolution of around 70 nanometers. In 2017, scientists improved the process by adding a second expansion step, achieving a total expansion of 20 times, which enables even higher resolution, though it makes the process more complex.
Scientists have developed several 20-fold expansion technologies requiring multiple expansion steps.
In the new study, the researchers aimed to achieve 20-fold expansion in just one step. This required finding a gel that was both highly absorbent and stable enough to maintain its structure when expanded.
They used a gel made from N, Dimethyl Acrylamide (DMAA), and sodium acrylate. Unlike previous gels, which needed an extra molecule to create crosslinks, this new gel forms crosslinks spontaneously and has strong mechanical properties. While earlier versions could only expand about tenfold, the MIT team optimized the gel and the polymerization process for robust 20-fold expansion.
To further stabilize the gel and improve its consistency, the researchers removed oxygen from the polymer solution before it solidified. This step, which involves running nitrogen gas through the solution, prevents side reactions that could disrupt crosslinking.
Once the gel is formed, specific bonds in the proteins that hold the tissue together are broken, and water is added to allow the gel to expand. After this expansion, researchers can label the target proteins in the tissue and then image them for analysis.
Tay Won Shin, PhD ’23’23, said, “This approach may require more sample preparation compared to other super-resolution techniques, but it’s much simpler when it comes to the actual imaging process, especially for 3D imaging. We document the step-by-step protocol in the manuscript so readers can read it easily.”
Using this technique, the researchers successfully imaged tiny structures within brain cells, including synaptic nanocolumns, clusters of proteins at neuronal synapses that help neurons communicate by releasing neurotransmitters like dopamine.
They also studied cancer cells, imaging microtubules—hollow tubes that provide cell structure and are important for cell division—mitochondria, which generate energy, and the organization of nuclear pore complexes, which control access to the cell nucleus.
The technique is now used to image glycans and carbohydrates on cell surfaces that help regulate environmental interactions. This method could also help study tumor cells, giving scientists new insights into protein organization within those cells.
The researchers believe that any biology lab can easily adopt this low-cost technique. It uses standard chemicals and common equipment, such as confocal microscopes and glove bags, which are typically available in most labs.
MIT graduate student Shiwei Wang said, “Our hope is that with this new technology, any conventional biology lab can use this protocol with their existing microscopes, allowing them to approach resolution that can only be achieved with very specialized and costly state-of-the-art microscopes.”
Journal Reference:
- Wang, S., Shin, T.W., Yoder, H.B. et al. Single-shot 20-fold expansion microscopy. Nat Methods (2024). DOI: 10.1038/s41592-024-02454-9

Leave a Reply