October 10, 2024 by David Danelski, University of California – Riverside
Collected at: https://techxplore.com/news/2024-10-scientists-catalytic-efficiency-pollution-hydrogen.html
Hydrogen-burning internal combustion engines offer great promise in the fight against climate change because they are powerful without emitting any earth-warming carbon. They can power heavy-duty trucks and buses and are suited for off-road and agricultural equipment and backup power generators, providing cleaner alternatives to diesel engines.
Yet they are not entirely clean. They emit nitrogen oxides during the high-temperature combustion process. Nitrogen oxides react with other compounds in the atmosphere to form harmful ozone and fine particulate matter, which aggravate our lungs and lead to long-term health problems.
Fortunately, UC Riverside scientists have discovered a low-cost method to significantly reduce this pollution from hydrogen engines by improving the efficiency of their catalytic converters.
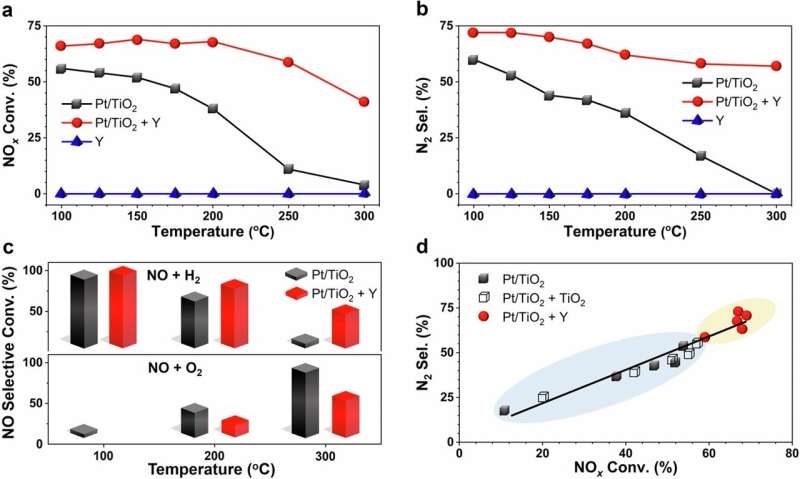
As reported in the journal Nature Communications, the researchers found that infusing platinum in catalytic converters with a highly porous material called Y zeolites greatly enhances the reactions between nitrogen oxides and hydrogen, converting them into harmless nitrogen gas and water vapor.
Compared to a catalytic converter without zeolites, the amount of nitrogen oxides converted to harmless substances increased by four to five times at an engine temperature of 250 degrees Celsius, the study found. The system was particularly effective at lower temperatures, which is crucial for reducing pollution when engines first start up and are still relatively cool.
What’s more, the technology can also reduce pollution from diesel engines equipped with hydrogen injection systems, explained Fudong Liu, the corresponding author and associate professor of chemical and environmental engineering at UCR’s Bourns College of Engineering. The hydrogen injection would be similar to the injection systems used in selective catalytic reduction systems for big-rig diesel trucks.
Zeolites are low-cost materials with a well-defined crystalline structure composed primarily of silicon, aluminum, and oxygen atoms. Their large surface area and three-dimensional, cage-like framework of uniform pores and channels allow for more efficient breakdown of pollutants.
By physically mixing platinum with Y zeolite—a synthetic type from the broader family of zeolite compounds—the researchers created a system that effectively captures water generated during the hydrogen combustion process. This water-rich environment promotes hydrogen activation, which is key to improving nitrogen reduction efficiency.
Shaohua Xie, a research scientist at UCR and lead author of the study, explained that the zeolite itself is not a catalyst. Instead, it enhances the effectiveness of the platinum catalyst by creating a water-rich environment. Liping Liu, a Ph.d. student, and Hongliang Xin, an associate professor at Virginia Tech, further validated this concept through theoretical modeling of the new catalyst system.
“This concept can also apply to other types of zeolites,” Xie added. “It’s a universal strategy.”
Liu emphasized that the pollution reduction method is relatively simple.
“We don’t need to use complicated chemical or other physical processes,” Liu said. “We just mix the two materials—platinum and zeolite—together, run the reaction, and then we see the improvement in activity and selectivity.”
UCR’s Liu, Xie, and Kailong Ye mixed powders of platinum and Y zeolite and provided them to collaborating scientist, Yuejin Li at BASF Environmental Catalyst and Metal Solutions (ECMS) in Iselin, New Jersey. The powder was made into a thick liquid slurry with binding compounds and applied to the honeycomb structures inside prototype catalytic converters. Scientists from National Synchrotron Light Source II, or NSLS-II, Brookhaven National Laboratory in Upton, New York, were also collaborators.
More information: Shaohua Xie et al, Zeolite-promoted platinum catalyst for efficient reduction of nitrogen oxides with hydrogen, Nature Communications (2024). DOI: 10.1038/s41467-024-52382-7
Journal information: Nature Communications

Leave a Reply