September 5, 2024 by University of Sheffield
Collected at: https://techxplore.com/news/2024-09-meta-analysis-paves-safer-batteries.html
Li-ion batteries can present major hazards, with the notion of safety based on narrow criteria. A meta-analysis of thermal runaway gas emissions by Sheffield researchers, published in the Journal of Energy Storage improves understanding and highlights the need for a broader analysis of risks.
A team from the Department of Chemical and Biological Engineering, led by Professor Sol Brown, conducted a detailed meta-analysis of 60 papers in order to understand more about the gases released by lithium-ion batteries during thermal runaway (overheating).
The main aim of the review was to see how different battery features affected the amount and type of gas released, such as battery design and how charged it is, and to see if they could discover what type of battery is the least hazardous in terms of fire and explosions.
Professor Sol Brown, Professor of Process and Energy Systems, said, “With the increase of electric vehicle sales and production, there is a concern that we do not have a strong understanding of the dangers they pose where they fail and ignite.
“Unlike gasoline and diesel fires, which have been the subject of ample research, Li-ion battery fires are comparatively understudied. This restricts our ability to effectively deal with them if they occur. To address this gap, the meta-analysis we have undertaken highlights some key findings and makes recommendations for further research to improve battery safety in the future.”
When abused, lithium-ion batteries overheat and can catch fire. Also during this process is the release of gases which can explode, and have the potential to cause serious injury. Further, some of the gases released, such as carbon monoxide and hydrogen fluoride, are poisonous and present a toxicity hazard.
However, beyond these broad characteristics, the findings of the research into gas emissions during thermal runway events are not clearly understood and need to be compared against each other.
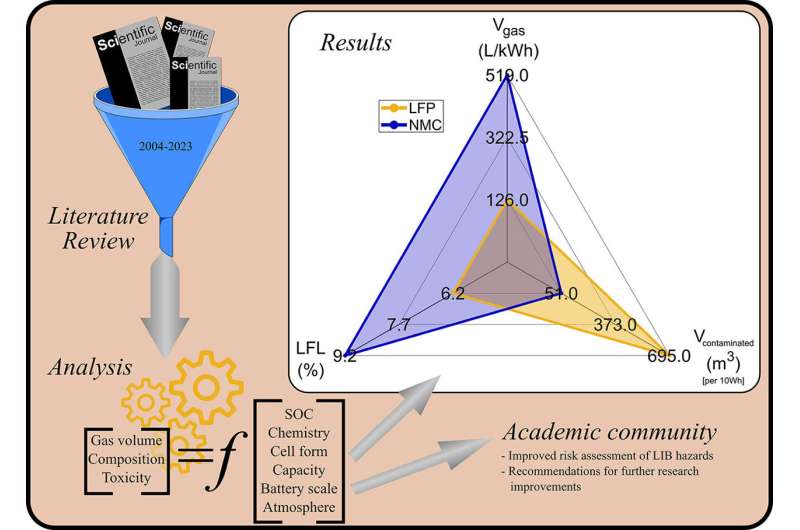
Most of the papers reviewed focused on lithium ion phosphate (LFP) and lithium nickel manganese cobalt oxide (NMC) batteries—both commonly used in EVs.
Key findings:
- The total amount of gas a battery releases increases as the battery gets bigger but the specific gases produced do not change.
- The shape of the battery matters—prismatic cells release more gas than pouch with cylindrical releasing the least.
- NMC batteries release more gas than LFP but LFP batteries are significantly more toxic
- Battery charge affects toxicity—for NMC batteries the contaminated volume doubles from 0% to 100% charge while for LFP it halves.
- LFP batteries produce more hydrogen whereas NMC produce more carbon monoxide. Researchers looked at something called the Lower Flammable Limit (LFL) to determine how likely the gas is to catch fire. The lower the LFL the easier it is for the gas to ignite. In an inert atmosphere the LFL levels are for LFP 6.2% and NMC 7.9% so LFP batteries present a greater flammability hazard.
The work in the paper aims to be a critical resource for the battery community to aid the risk assessment of lithium-ion battery thermal runaway fire, explosion and toxicity hazards.
The paper includes a number of recommendations so that significant improvements in research can be made to advance the understanding of lithium-ion off-gas further, including:
- More reporting on the material makeup of electrodes and the composition of the electrolytes inside NMC batteries. This will help us better understand if and how they affect the gases released.
- In order to get more accurate comparisons between high energy LFP and NMC batteries, more testing should be done on a wider range of LFP pouch and prismatic cells (10–100 Ah).
- Testing larger battery systems—current research focuses on single battery cells, but in real-world applications multiple cells are connected together, so it would be helpful to see how hazards scale with battery size.
- Measuring gas released at different charge levels—so far studies have only looked at fully charged batteries. It would be useful to see how toxicity and flammability of gas changes at different charge levels, especially when the battery is overcharged
- Future experiments should record how much and the type of electrolyte that is ejected as a vapor, as this would help assess additional fire risks.
More information: Peter J. Bugryniec et al, Review of gas emissions from lithium-ion battery thermal runaway failure — Considering toxic and flammable compounds, Journal of Energy Storage (2024). DOI: 10.1016/j.est.2024.111288

Leave a Reply