August 19, 2024 by Max Planck Society
Collected at: https://phys.org/news/2024-08-precisely-effect-electromagnetic-shielding-beryllium.html
The electron shell of atoms acts as an “electromagnetic shield,” preventing direct access to the nucleus and its properties. A team in the group of Klaus Blaum, director at the Max Planck Institute for Nuclear Physics in Heidelberg, has now succeeded in precisely measuring the effect of this shielding in beryllium atoms. The study is published in the journal Nature.
The magnetic moment of beryllium-9 was determined with 40 times better precision than before. Such precision measurements are not only relevant to fundamental physics, they also help us gain insight into certain applications of nuclear magnetic resonance, which are applied in chemistry and for the highly accurate measurements of magnetic fields.
“Shields up!”: This command is familiar to fans of “Star Trek.” Something similar is known to natural science researchers—the electromagnetic shell serves as a protective shield that typically hinders access to its nucleus. This has consequences in chemistry, for example, where chemical properties are investigated by nuclear magnetic resonance.
This method is akin to magnetic resonance imaging. However, instead of producing images of a living body, it provides a highly precise chemical fingerprint of the material being studied. Both methods use strong magnetic fields and base on the fact that some nuclei are small magnets—like tiny compass needles.
In a strong magnetic field, they can start to spin in a circular motion. Like in a class room experiment with an induction coil through which a magnet is moved, this movement of the atom interacts with the surrounding electron shell. As the electrons make up the chemical bonds, the signal of the precessing atomic nuclei gives very precise information on their chemical surroundings.
The three-body problem
Now, one might think that in modern physics, the magnetic moment of a nucleus and the shielding effect of the electron shell could be calculated accurately. However, this is not the case, confirms Zoltan Harman, who is responsible for such theoretical calculations at the institute in Heidelberg. The reason—as is often the case—is the fundamental problem that calculations for systems consisting of more than two bodies can not be performed exactly.
This applies to planetary orbits in stellar systems as well as to atoms, whose electrons can only be in certain quantized energy orbitals around the nucleus. In addition, an atomic nucleus itself cannot be calculated exactly. Even the simplest nucleus, the single proton in hydrogen, consists of three quarks that interact with each other in complex ways.
“Theoreticians can therefore only calculate such a nuclear moment to an uncertainty of about one per thousand,” says Stefan Dickopf. For applications in nuclear magnetic resonance and fundamental physics, high-precision experiments are therefore important in order to measure such properties much more accurately than calculations can.
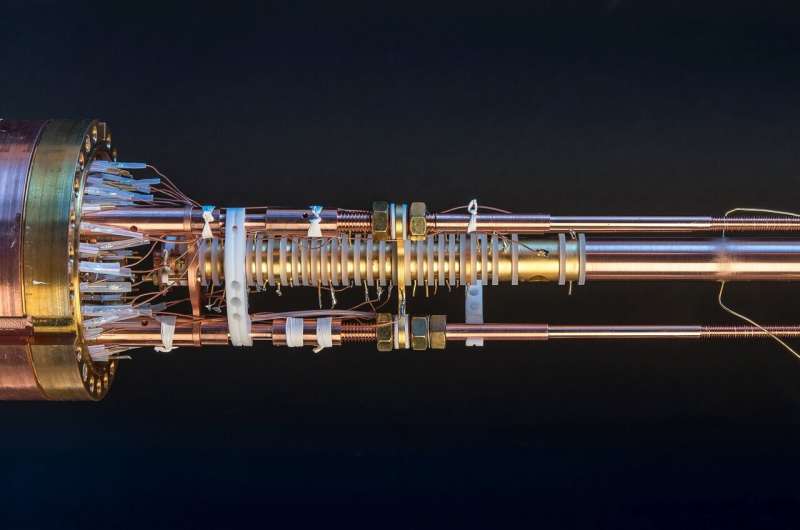
Klaus Blaum’s team has developed a method with world-class performance using so-called Penning traps. It makes it possible to measure the magnetic properties of an atomic nucleus very precisely. Stefan Dickopf, the lead doctoral student in the team headed by Andreas Mooser, has now carried out such measurements on the isotope beryllium-9.
More information: Stefan Dickopf et al, Precision spectroscopy on 9Be overcomes limitations from nuclear structure, Nature (2024). DOI: 10.1038/s41586-024-07795-1
Journal information: Nature

Leave a Reply