JULY 24, 2024 by Eberhard Fritz, Max Planck Society
Collected at: https://phys.org/news/2024-07-net-effects-nitrogen-attenuate-global.html
Nitrogen fertilizers and nitrogen oxides from fossil fuels are known for their environmental damage: they pollute the air and drinking water, lead to over-fertilization of water and land ecosystems, reduce biodiversity and damage the ozone layer.
As far as climate is concerned, however, they have a net cooling effect. This is the conclusion reached by an international team led by scientists from the Max Planck Institute for Biogeochemistry in Jena in a comprehensive analysis. In it, the scientists take stock of the various climate effects of nitrogen compounds from agricultural and non-agricultural sources.
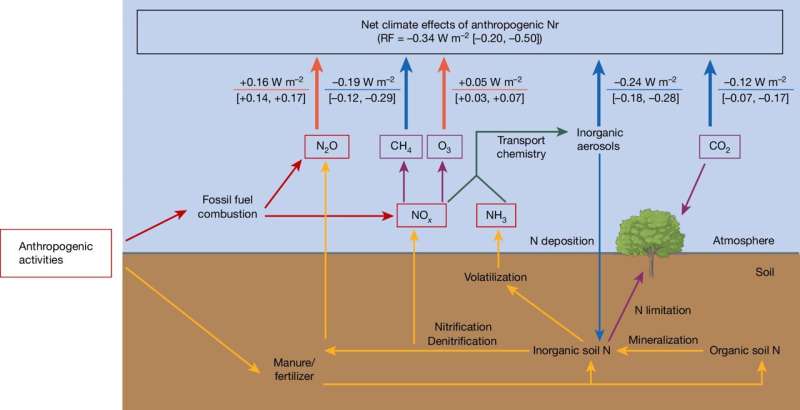
Nitrogen exists as diverse forms in soil, water and air, and thus affects climate through various pathways. Elemental nitrogen, which makes up around 78% of air, is climate-neutral, but all other nitrogen compounds, known in science as reactive nitrogen, can have direct or indirect effects on the global climate—sometimes warming and sometimes cooling.
For example, nitrous oxide, commonly known as laughing gas, which is mainly emitted from nitrogen-rich soils and fossil fuel combustion, is almost 300 times more potent a greenhouse gas than CO₂ and much longer-lived in the atmosphere.
By contrast, the short-lived nitrogen oxides mainly produced by the combustion of fossil fuels form fine suspended particles in the atmosphere. They shield sunlight and thus cool the climate. The same holds true for ammonia, which is among others resulting from the application of liquid manure and artificial fertilizers.
At the same time, nitrogen deposited on land allows plants to grow more abundantly and thus absorb more CO₂ from the atmosphere, which also has a cooling effect. Nitrogen oxides also play a role in the breakdown of atmospheric methane and they thus cool the atmosphere. However, they also stimulate the formation of ozone in the troposphere, which is a potent greenhouse gas and enhances anthropogenic global warming.
Without man-made nitrogen input, the climate would have heated up even more
The international team, led by Sönke Zaehle and Cheng Gong from the Max Planck Institute for Biogeochemistry, has now summarized the various warming and cooling effects, in a study which has been published in the journal Nature. They found that reactive nitrogen, which enters the Earth system through human activities, cools the climate by an amount of -0.34 watts per square meter—in climate research, this is referred to as a net negative radiative forcing.
By comparison, man-made global warming heats the atmosphere with an additional 2.7 watts per square meter, mainly due to greenhouse gases from fossil fuels—this is the average value for the years 2011 to 2020, as stated by the Intergovernmental Panel on Climate Change (IPCC) in its latest Assessment Report. At the same time, Earth has warmed by 1.1 degrees Celsius in this period compared to pre-industrial times.
“The negative radiative forcing due to man-made nitrogen inputs cannot simply be converted into a change in global mean temperature, as some effects occur locally and the climate system reacts in a complex way to such changes in radiative forcing,” says Zaehle, co-author of the study and director at the Max Planck Institute for Biogeochemistry.
However, despite the warming effect from nitrous oxide, the new study makes it clear that the climate would have heated up even further without human nitrogen input.
“This may sound like good news, but you have to bear in mind that nitrogen emissions have many harmful effects, for example on health, biodiversity and the ozone layer,” says Zaehle. “The current findings therefore are no reason to gloss over the harmful effects, let alone see additional nitrogen input as a means of combating global warming.”
The scientists determined the overall impact of nitrogen from human sources by first analyzing the quantities of the various nitrogen compounds that end up in the soil, water or air. They fed this data into models from the NMIP2 project that depict the global nitrogen cycle and the effects on the carbon cycle, i.e. the stimulation of plant growth and ultimately the CO₂ and methane content of the atmosphere.
From the results of these model simulations, they used another atmospheric chemistry model to calculate the effect of man-made nitrogen emissions on radiative forcing, that is the radiant energy that hits one square meter of the Earth’s surface per unit of time.
“Previous estimates based on literature studies were usually fragmentary and therefore neglected the fact that the processes of the global nitrogen cycle are spatially very heterogeneous, highly interconnected and non-linear,” says Gong, postdoc at the Max Planck Institute for Biogeochemistry and first author of the study.
“Our results emphasize how important it is to consider the interactions between biogeochemistry, atmospheric chemistry and climate in order to understand the climate impact of anthropogenic nitrogen.”
Alleviating nitrogen pollution should be backed up with even stronger efforts in reducing fossil-fuel greenhouse gases
“Nitrogen emissions should be reduced,” says Zaehle. “Improved agricultural practices could help to use nitrogen as a fertilizer more efficiently. In this way, for example, nitrous oxide emissions, which contribute to global warming and damage the ozone layer, can be reduced.
“However, it is important to recognize that while reducing anthropogenic nitrogen inputs benefits human health and ecosystems, it also has an impact on the climate. In addition to reducing reactive nitrogen, emissions of greenhouse gases, especially CO₂ and methane from fossil fuels, must therefore also be reduced to a greater extent. Only then can we protect health and nature better and mitigate climate change.”
More information: Cheng Gong, Global net climate effects of anthropogenicreactive nitrogen reactive nitrogen, Nature (2024). DOI: 10.1038/s41586-024-07714-4. www.nature.com/articles/s41586-024-07714-4
Journal information: Nature

Leave a Reply