JUNE 20, 2024 by ICFO
Collected at: https://techxplore.com/news/2024-06-catalyst-unveils-hidden-power-green.html
Hydrogen is a promising chemical and energy vector to decarbonize our society. Unlike conventional fuels, hydrogen utilization as a fuel does not generate carbon dioxide in return. Unfortunately, today, most of the hydrogen that is produced in our society comes from methane, a fossil fuel. It does so in a process (methane reforming) that leads to substantial carbon dioxide emissions. Therefore, the production of green hydrogen requires scalable alternatives to this process.
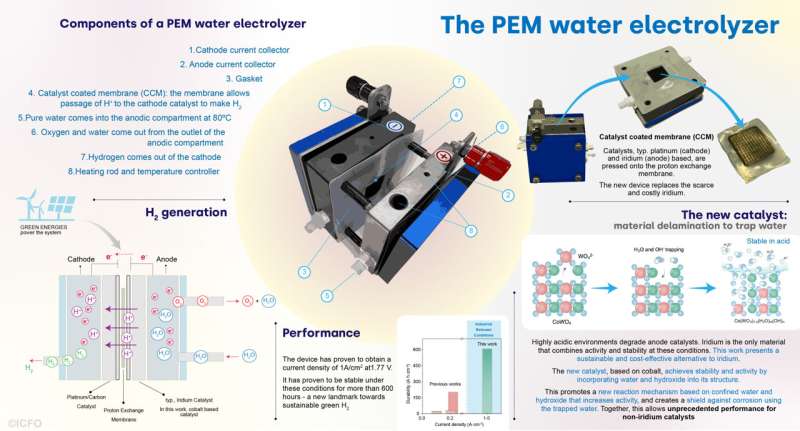
Water electrolysis offers a path to generate green hydrogen which can be powered by renewables and clean electricity. This process needs cathode and anode catalysts to accelerate the otherwise inefficient reactions of water splitting and recombination into hydrogen and oxygen, respectively. From its early discovery in the late 18th century, water electrolysis has matured into different technologies. One of the most promising implementations of water electrolysis is the proton-exchange-membrane (PEM), which can produce green hydrogen combining high rates and high energy efficiency.
To date, water electrolysis—and in particular PEM—has required catalysts based on scarce, rare elements, such as platinum and iridium, among others. Only a few compounds combine the required activity and stability in the harsh chemical environment imposed by this reaction. This is especially challenging in the case of anode catalysts, which have to operate in highly corrosive acidic environments—conditions where only iridium oxides have shown stable operation at the required industrial conditions. But iridium is one of the scarcest elements on Earth.
In the search for possible solutions, a team of scientists has recently taken an important step to find alternatives to iridium catalysts. This multidisciplinary team has managed to develop a novel way to confer activity and stability to an iridium-free catalyst by harnessing so far unexplored properties of water. The new catalyst achieves—for the first time—stability in PEM water electrolysis at industrial conditions without the use of iridium.
This breakthrough, published in Science, has been carried out by ICFO researchers Ranit Ram, Dr. Lu Xia, Dr. Anku Guha, Dr. Viktoria Golovanova, Dr. Marinos Dimitropoulos, Aparna M. Das and Adrián Pinilla-Sánchez, and led by Professor at ICFO Dr. F. Pelayo García de Arquer; and includes important collaborations from the Institute of Chemical Research of Catalonia (ICIQ), The Catalan Institute of Science and Technology (ICN2), French National Center for Scientific Research (CNRS), Diamond Light Source, and the Institute of Advanced Materials (INAM).
Dealing with the acidity
Combining activity and stability in a highly acidic environment is challenging. Metals from the catalyst tend to dissolve, as most materials are not thermodynamically stable at low pH and applied potential in a water environment. Iridium oxides combine activity and stability in these harsh conditions, and that is why they are the prevalent choice for anodes in proton-exchange water electrolysis.
The search for alternatives to iridium is not only an important applied challenge, but a fundamental one. Intense research in the search for non-iridium catalysts has led to new insights into the reaction mechanisms and degradation, especially with the use of probes that could study the catalysts during operation combined with computational models. These led to promising results using manganese and cobalt oxide-based materials, and exploiting different structures, composition, and dopants, to modify the physicochemical properties of the catalysts.
While insightful, most of these studies were performed in fundamental not-scalable reactors and operating at softer conditions that are far from the final application, especially in terms of current density. To date, demonstrating activity and stability with non-iridium catalysts in PEM reactors and at PEM-relevant operating conditions (high current density) had remained elusive.
To overcome this, the ICFO, ICIQ, ICN2, CNRS, Diamond Light Source and INAM researchers came up with a new approach in the design of non-iridium catalysts, achieving activity and stability in acid media. Their strategy, based on cobalt (very abundant and cheap), was quite different from the usual paths.
“Conventional catalyst design typically focuses on changing the composition or the structure of the employed materials. Here, we took a different approach. We designed a new material that actively involves the ingredients of the reaction (water and its fragments) in its structure. We found that the incorporation of water and water fragments into the catalyst structure can be tailored to shield the catalyst in these challenging conditions, thus enabling stable operation at the high current densities that are relevant for industrial applications,” explains Professor at ICFO García de Arquer.
With their technique, consisting of a delamination process that exchanges part of the material for water, the resulting catalyst presents as a viable alternative to iridium-based catalysts.
More information: Ranit Ram et al, Water-hydroxide trapping in cobalt tungstate for proton exchange membrane water electrolysis, Science (2024). DOI: 10.1126/science.adk9849. www.science.org/doi/10.1126/science.adk9849
Journal information: Science

Leave a Reply