DECEMBER 23, 2021 by David Appell , Phys.org
Mercury is a most unusual planet. The smallest planet in the solar system, and the closest planet to the sun, it is in a 3:2 spin resonance, slowly turning and experiencing scorching heat up to 430 degrees Celsius, and the night side frigid, down to -170 degrees Celsius. Due to its much larger iron-rich core compared to Earth, it has the second-highest average density in the solar system, just 1.5 percent below Earth’s. Despite its proximity to the sun, the surface of Mercury was, surprisingly, found to be rich in volatile elements such as sodium and sulfur.
Notably, the planet’s separation into an iron-rich core and rocky mantle (the geological region between the core and the crust) suggests Mercury had a magma ocean early in its formation. Like any liquid, this ocean would have evaporated, but in the case of Mercury, the temperatures were likely to have been so high that the vapor was not composed of water, but rock. In a new study published in The Planetary Science Journal, Noah Jäggi and colleagues modeled how the evaporation of the surface of this magma ocean would form an atmosphere and determined whether losses from the atmosphere could alter Mercury’s composition, addressing an open question of why moderately volatile elements like sodium have accumulated on Mercury’s surface. Their results were surprising, Jäggi, a graduate student at the University of Bern, told Phys.org.
Early planetary magma oceans aren’t unusual, explained Lindy Elkins-Tanton, director of the School of Earth and Space Exploration at Arizona State University. “We think all rocky planets have one or more—maybe several—magma oceans as they form. The impacts of accretion toward the end of planet formation are just that energetic; they will melt the planets to some depth.”
The early solar system was a rough and active place, full of flying rocks, massive collisions and heavy bombardments. The heat these events generated, in addition to radioactive decay and heat produced by gravitational setting of Mercury’s iron-rich core, kept the planet’s surface and interior molten. Models indicate these processes caused the temperature of the surface to rise to about 2,400 K (3,860 degrees Fahrenheit). Could evaporation and then atmospheric loss change the makeup of Mercury?
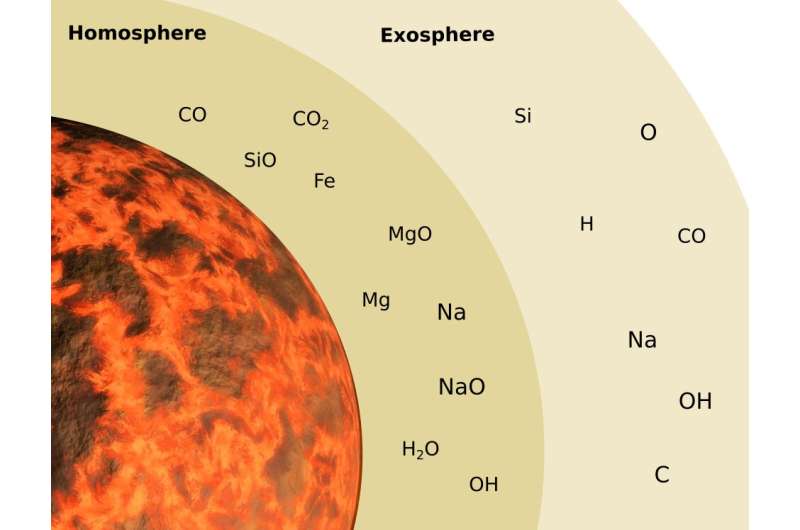
Jäggi and his team assumed two initial sizes for Mercury, one larger than today’s, as some scientists hypothesize, and four possible magma ocean compositions. Volatile species like carbon dioxide, carbon monoxide, hydrogen (H2) and water dissolve in magma and can escape as a gas when pressure is released. Comparably nonvolatile, rock-forming elements such as silicon, sodium or iron can exist as gases such as silicon monoxide (SiO) only at the very high temperatures thought to have existed in the early magma ocean. The difference between the volatile and nonvolatile gaseous species is that, for a given temperature, volatile species have much larger equilibrium vapor pressures than do nonvolatiles. This is the pressure the atmosphere exerts at the atmosphere-magma surface when the two coexist.
The research team ran a coupled interior-atmospheric model to determine the effect of evaporation from the ocean into the atmosphere, and after accounting for atmospheric chemical and physical processes, the resulting mass loss from the atmosphere either to space or back to the planet. Meanwhile, the planet was cooling. Liquid magma begins to crystallize at 1,700 K (2,600 degrees Fahrenheit) which makes 1,500 K used by Jäggi a good approximation for the lifetime of the surface melt and sets the end point for mass loss sourced by the magma ocean of Mercury.
In both the volatile and nonvolatile case, the magma ocean evaporates to supply the atmosphere. Molecules can escape the atmosphere in one of four ways—plasma heating from the solar wind of charged particles; photoevaporation of atmospheric species from extremely high-energy solar photons such as X-rays and ultraviolet photons from the sun deep in the upper atmosphere creating an outflow of gas (also called hydrodynamic escape); Jeans escape, where especially high-altitude, high-velocity, low-mass molecules zip out the top of the atmosphere before encountering another molecular collision; and photoionization, where high-energy photons produce ions that escape via various means.
The team’s model found that of the four potential escape mechanisms, Jeans escape was negligible, with the others leading to mass losses from 1 million to 4 billion kilograms per second, depending on the timing of Mercury’s formation and assumptions about heating efficiencies, with the upper range coming from hydrodynamic escape—”from insignificant to predominant,” said Jäggi, depending on how efficiently atmospheric species are heated and how much radiation was produced and delivered by the early sun.
But importantly, the total loss of mass from the two vastly differently atmospheres tested—volatile and nonvolatile—were found to be quite similar. Given the mass loss, the model’s resulting timescale for efficient interior-atmosphere chemical exchange was less than 10,000 years, implying atmospheric escape processes only account for about 0.3 percent of Mercury’s initial mass, or less than 2.3 kilometers of crust. (Mercury’s present-day radius is 2,440 km.)
So cumulative mass loss seems not to have significantly modified Mercury’s bulk mantle composition during the magma ocean stage. Thus, the cooling times, which depend on the greenhouse effect induced, determined how much material is lost over the lifespan of the magma ocean.
The insignificance of the total atmospheric mass loss from Mercury, hydrodynamic escape aside, was surprising, Jäggi said. “It tells us that there must be more to the high sodium measurements on Mercury’s surface, as they cannot be accumulated nor lost in any significant amount given our modeled loss rates and magma ocean lifetimes.” The results could be extended to the moon, an exoplanet or Earth-like planet that begins in a hot magma phase “with a volatile budget delivered by its building blocks.”
More information: Noah Jäggi et al, Evolution of Mercury’s Earliest Atmosphere, The Planetary Science Journal (2021). DOI: 10.3847/PSJ/ac2dfb
Journal information: The Planetary Science Journal
